- Your cart is empty
- Continue Shopping
Copper treatment – 14 days or 30 days Treatment Method
Copper treatment – 14 days or 30 days Treatment Method
Taking the Hanna copper checker’s margin of error into consideration, we are now recommending 2.2-2.3 ppm for Copper Power. This ensures that the copper level is “just right” i.e. not too high, not too low.
Copper treatment (updated 5-16-2023)
What It Treats: Marine Ich (Cryptocaryon irritans) and Marine Velvet Disease (Amyloodinium ocellatum). There is some anecdotal evidence that copper will sometimes suppress symptoms of Brooklynella and Flukes (Monogeneans); however copper is unlikely to completely eradicate either.
How To Treat: First, it is important to know what type of copper you are using. Below is a list of the most commonly available copper products, their therapeutic level and alternative copper test kit(s) that can be used if applicable. With all copper products, it is best to test using the Hanna High Range Copper Colorimeter HI702.
- Copper sulfate (0.20 ppm): Seachem or Salifert copper test kit
- Cuprion (0.20 ppm): Seachem or Salifert copper test kit
- Cupramine (0.5 ppm): Seachem or Salifert copper test kit
- Coppersafe (2.0 ppm): No suitable alternative – Only use the Hanna checker
- Copper Power* (2.2-2.3 ppm): No suitable alternative – Only use the Hanna checker
* Copper Power Dosing Calculators: calconic.com OR fishotel.com
Starting Point: Copper sulfate, Cuprion and Cupramine should all ideally be ramped up slowly, taking several days to reach therapeutic. (Obviously, this is not always practical when treating an active outbreak of Ich or Velvet.) However, with chelated copper (Coppersafe or Copper Power) you are able to drop most fish straight into 2.0 ppm. I then recommend taking ~ 48 hours to reach 2.2-2.3 ppm when using Copper Power.
There is no “ramping down” period that is necessary with copper treatment. Meaning, a fish can go from full therapeutic to zero copper without experiencing any issues.
Treatment timeframes: The first thing you need to know is that the “copper clock” does not start until you have reached a therapeutic level (see above). It is also important to treat at “reef temperatures” (77-80F) so the lifecycle of the parasites proceed as expected. The traditional way of using copper is 30 days at a therapeutic level in a bare bottom QT. A therapeutic level must be maintained at all times during the 30 days, so testing often is important. If the level drops even slightly out of range, then the 30 day clock restarts. One reason your copper level may drop unexpectedly is if you are treating in a tank with rock or other material which absorbs copper. Conversely, if you exceed the therapeutic level then you risk killing the fish. At the end of 30 days, remove all copper via water changes, Cuprisorb, Polyfilter, etc. and observe the fish for 2-4 weeks to ensure treatment was successful.
An alternative way to use copper is to treat for just 2 weeks and then transfer the fish to a different observation tank. However, adhering to the following “rules” is very important if you wish to try this:
- Copper level must be at FULL THERAPEUTIC (2.2-2.3 ppm if using Copper Power) for the entire 14 days (very important).
- QT water temp should stay consistently between 77-80F.
- Nothing from the QT can be reused to setup the observation tank. Transfer just the fish, nothing else!
- The two tanks should be at least 10 feet apart, to avoid any possibility of aerosol transmission. Also be careful to avoid cross contamination via wet hands, feeding apparatus, anything wet really…
- Do not lower the copper level prior to transferring. The observation tank should be copper free, so you can observe to ensure treatment was successful. You can, however, treat with other medications (e.g. Praziquantel if you need to deworm) during observation.
The above strategy works because after 14 days any ich/velvet trophonts should have dropped off a fish, and were unable to reattach… Provided you treated: a) With therapeutic copper the entire time b) At “reef temperatures” (77-80F). The presence of therapeutic copper in the water shields your fish from reinfection from any unhatched tomonts (which release free swimmers). It’s these unhatched tomonts you are transferring your fish away from by utilizing this method. Therefore, understand that the QT is still possibly contaminated with ich and/or velvet tomonts even after all the fish have been transferred out. Which is why sterilizing your QT in-between batches of fish is always a good idea.
Pros: Readily available, proven treatment.
Cons/Side Effects: Copper is a poison, pure and simple. It only works because most fish are able to withstand being in it longer than the parasites. Appetite suppression and lethargy are common side effects. If a fish’s appetite lessens, that is usually OK. But when a fish stops eating entirely this means that you’ve likely encountered a “copper sensitive” fish and an alternative treatment should be used instead. source
More info: Use of Copper in Marine Aquaculture and Aquarium Systems
The most notable update is I am now recommending a therapeutic level of 2.5 ppm when using Copper Power. This is in line with the manufacturer’s recommendation for combating “copper resistant parasites”.
14 days in copper method
Basically, you treat a fish for 14 days at a therapeutic copper level (VERY IMPORTANT DETAIL) and then transfer the fish into a different (non-medicated) observation tank. The devil is in the details with this method, so please observe the following “rules” whenever using this approach:
- Only the fish gets transferred, nothing else. Meaning, do not use ANYTHING from the treatment tank to setup the observation tank. You must use a new tank + all new equipment to setup the observation tank to avoid cross contamination. (Specifically, unhatched tomonts.)
- DO NOT lower the copper level prior to transferring. Fish can safely be transferred from full therapeutic to no copper in the water. The small amount of copper water on the fish or net is negligible.
- The observation tank must be at least 10 feet away from the QT/treatment tank, Display Tank and all other saltwater aquariums. This is to avoid aerosol transmission: Aerosol transmission
- The 14 day countdown does not begin until copper has reached therapeutic, and has been maintained at therapeutic throughout (very important!) for the 14 days. So, it is wise to test your copper level daily. The following are therapeutic copper levels for various products:
- Copper sulfate or Cuprion: 0.20 ppm
- Cupramine: 0.5 ppm
- Coppersafe*: 2.0 ppm
- Copper Power*: 2.0 – 2.5 ppm
* Read this regarding Copper Power/Coppersafe and also click here for a Copper Power Dosing Calculator.
One last thing: Prior to transfer, the fish should not be showing any signs of ich, Velvet, Brooklynella or Uronema. If the fish does, DO NOT do the transfer!
How do I test my copper level?
Nowadays, everyone using copper should invest in an accurate Hanna High Range Copper Colorimeter (HI702): High Range Copper Colorimeter – Checker® HC HI702
The other copper test kits are inaccurate and rely too much on the subjectivity of you being able to read a color chart. (Some of us are colorblind and don’t even realize it!)
How does the 14 days in copper method work??
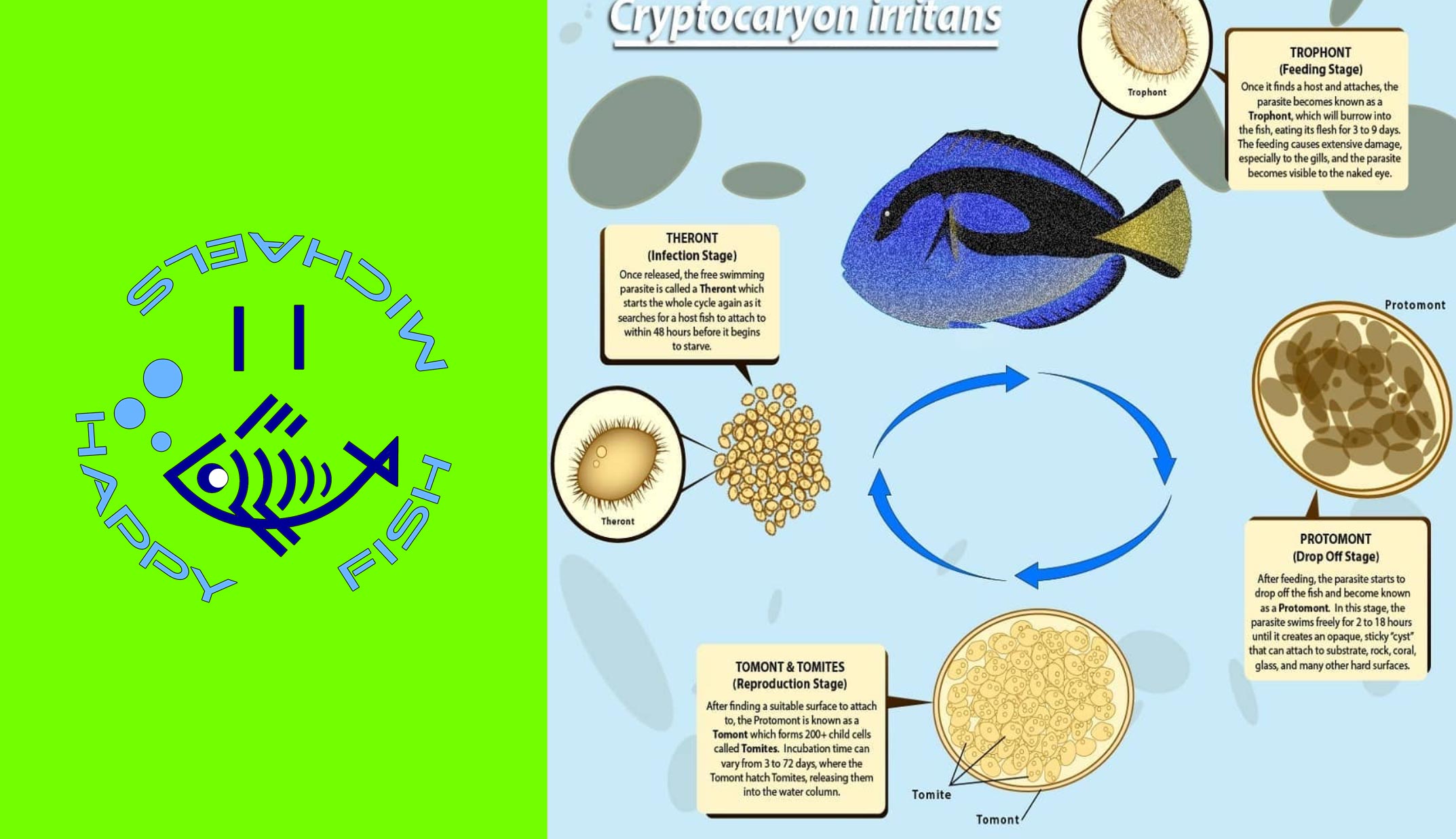
Copper only treats two parasites: Ich & Velvet. Velvet has a much shorter lifecycle than Marine Ich, so let’s focus on Ich’s lifecycle:
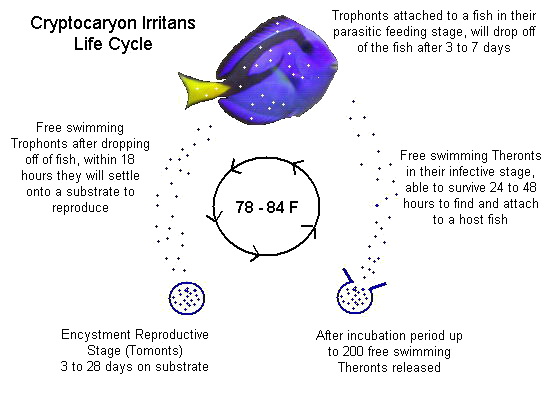
1. So long as you are treating above 76F, Ich trophonts can only remain on a fish for a maximum of 7 days.
2. Even in cool water studies (low 70s), no trophont has ever been documented to stay on a fish for longer than 14 days.
3. Regardless of temperature, I still recommend 14 days in therapeutic copper before transferring (out of an abundance of caution.)
4. Copper kills one (and only one) life stage of Ich & Velvet: The free swimming Theront stage (called dinospores with Velvet).
So, once all the trophonts have dropped off a fish the therapeutic copper in the water is acting like a shield to protect the fish from reinfection. Contact with therapeutic copper water either kills or disables/sterilizes any free swimmers in the water. The free swimmers will die before infecting a fish or even if they do manage to latch on for a few hours they are essentially doomed. They cannot reproduce and continue on with the parasite’s lifecycle. Therefore, by keeping a fish in therapeutic copper for 14 days and then transferring into an observation tank there is minimal chance of free swimmers being able to reinfect the fish. The primary benefit of this approach (vs. 30 days in copper) is to get the fish out of copper (a liquid poison) as soon as possible.
Can the 14 days in copper approach fail??
Sure it can! After all, no treatment or quarantine protocol is 100% foolproof! However, reasons for failure are usually one of the following:
- Failure to maintain therapeutic copper for 14 continuous days! People get lazy, stop testing, assume the copper is at therapeutic, but something in the QT is absorbing the copper and it drops below therapeutic. Subtherapeutic copper does not have a 100% kill rate!
- Cross contamination (e.g. water hoses, equipment, feeding apparatus) between the observation tank and the QT, or another infected aquarium like your frag tank. Also, aerosol transmission is sometimes to blame: Aerosol transmission
- Trying to reuse equipment from the QT to setup the observation tank. This is a risk not worth taking. Just buy all new equipment for the observation tank.
- Encountering so-called copper resistant parasites, which is still unproven at this time of writing. In theory, parasites which have spent years in subtherapeutic copper could build up a resistance to even therapeutic copper. However, a failure from this would likely happen in both a 14 or 30 day copper QT environment. The only option would be to treat using a different method: Chloroquine for Ich/Velvet, or Tank Transfer Method or Hyposalinity for Marine Ich only. Also look into “Hybrid” Tank Transfer Method: Hybrid TTM to treat all parasites!
- Therapeutic copper does not kill or disable ALL of the free swimmers in a single pass / following just one exposure. Copper gets the rest on the second pass. Again, another unproven theory. Basically, the belief is that copper only weakens (but doesn’t outright kill) some of the free swimmers, but these “damaged but still viable” parasites become more susceptible to copper during the next encounter. After they’ve fed, dropped off the fish, formed tomonts and released (weaker) free swimmers into the water. I don’t even know what to think about this, or how you would ever know for sure that such parasites would eventually be killed off.
What’s wrong with treating for 30 days in copper??
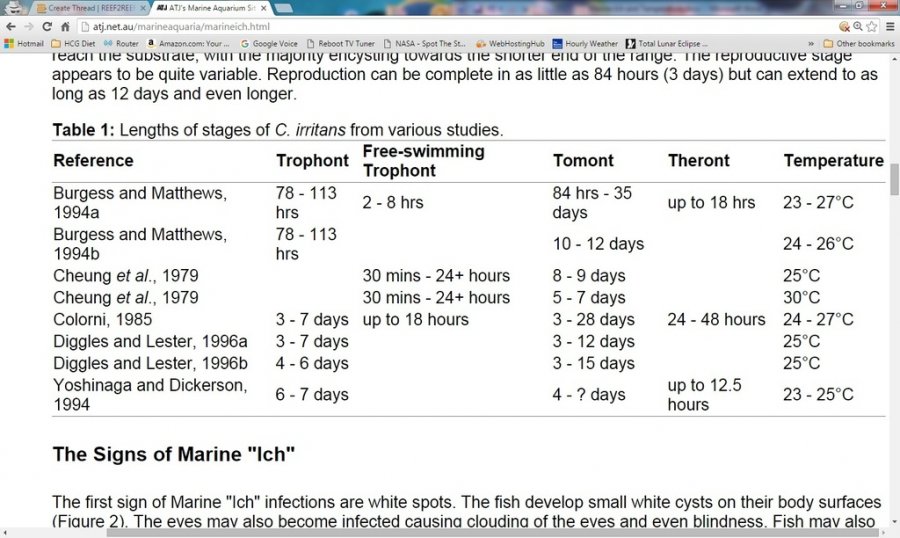
Nothing really! Except some fish cannot last in copper for that long, AND there have been at least a couple of studies (below) where it took longer than 30 days for all of the theronts to be released from their tomonts. So, 42-45 days in therapeutic copper would actually be a safer number if using just 1 QT (no transfer).
Post treatment observation is King!
Whether you are treating with copper for 14 days, 30 days or even 45 days… You should always observe the fish in non-medicated water for 2 to 4 weeks before placing in your DT! No treatment is bulletproof, mistakes can and will happen, Murphy’s law and all that jazz. The biggest mistake I see people make is transferring fish straight from a medicated QT into their DT. You have no idea whether or not the treatment(s) you applied were successful. Also, copper DOES NOT TREAT Brooklynella, Uronema, Flukes, Turbellarians, bacterial diseases, etc. So, you’ll want to either prophetically treat for these in the observation tank or at least watch for symptoms of these diseases. I highly recommend using black mollies in observation to aid with detecting diseases: Black Molly Quarantine
Some say black mollies don’t work, but I don’t know what the downside is of having more fish to watch for diseases in observation.
P.S. The 14 day and transfer method should also work with Chloroquine IF you maintain a therapeutic level (at least 40mg/gal) throughout: Chloroquine Phosphate
source
some prefer 14 days and transfer for a couple of reasons:
- Some fish struggle in copper from Day 1. Or start showing signs of copper intolerance at some point along the way. From my way of thinking, those fish are much more likely to survive 14 days vs. 30 days in copper.
- I feel 14 days is more effective because you are transferring the fish away from any unhatched tomonts in the original QT. As previously noted, in some rare cases 42-45 days worth of therapeutic copper may be necessary to be completely sure all Ich tomonts are no longer viable in the QT. 45 days in copper?!? F that.
caution with 14 days
there is a good chance that parasite tomonts may still be encysted somewhere in the tank after only 2 weeks. If wanting to use a single aquarium for both QT + observation, the best strategy is to treat with therapeutic copper (or chloroquine) for 30 days before lowering it.
14 days only works if you have a second completely sterile tank to transfer the fish into after 14 days in copper. I do prefer the 14 day method as copper is an immunosuppressant. So the quicker you can get them out of copper the better.
USE OF COPPER IN MARINE AQUACULTURE AND AQUARIUM SYSTEMS
INTRODUCTION
Copper has been used effectively for many years to control algae and fish parasites in freshwater and marine systems. Because copper does not discolor the water, it is a preferred treatment for use in display aquaria. Water chemistry and other environmental factors will determine how much copper will be biologically available and for how long.
However, the copper concentrations required for effective treatment may be acutely toxic for some species of finfish and are lethal for most invertebrates. Chronic copper exposure will also adversely affect fish health. Sublethal and toxic levels of copper damage gills and other tissues of fish, and also are known to depress the immune system. Because of all these concerns, it is important to understand how copper works and how copper availability is affected by the environment in which it is used (Cardeilhac and Whitaker 1988).
Calculations and follow up procedures required for the use of copper in marine systems are different from the calculations and procedures you would use for copper in freshwater (Watson and Yanong 2006). Factors including parasite life cycle, susceptibility and non-target species sensitivities will also factor in your determination of whether or not to use copper and, if you do use it, how long to continue the treatment to ensure it is both effective and safe.
This publication will concentrate on the use of “bluestone” or “blue copperas” copper sulfate (CuSO4 •5H2O; i.e., copper sulfate pentahydrate), the most commonly used form of copper for aquaculture. However, before you use copper or any other chemical or drug, be sure to review local, state, and federal regulations and guidelines for legalities regarding application and discharge of the treated water.
BASIC COPPER CHEMISTRY
Copper is a heavy metal that can be found naturally in a number of different forms. The form of copper that is most effective for algae and parasite control is the positively charged copper with a 2+ charge, also known as “Cu2+.” This is the form that is found in “bluestone” copper sulfate (more properly known as “copper sulfate pentahydrate” because it is attached to 5 water molecules).
When copper sulfate is dissolved into water, copper sulfate splits into separate copper (Cu2+) and sulfate (SO42-) ions (and water). Because this “Cu2+” is the “active ingredient” of “bluestone” copper sulfate, this is the ion that must remain in solution and which must be measured. For susceptible marine parasites, including Amyloodinium (Reed and Floyd 1994) and Cryptocaryon (Yanong 2009), the target concentration is 0.15–0.20 mg/L Cu2+.
Maintaining target concentration levels of copper can be challenging. Keeping copper concentrations high enough is difficult for many reasons. Water has numerous dissolved compounds (for example, bicarbonate ion (HCO3–), which can readily “combine” with copper and remove copper from solution. Carbonates—which are part of dolomite, crushed coral, oyster shell, and other common marine substrates—dissolve in the water and complex (or bind) with copper, affecting the level of copper in solution. Copper can also be taken up by living organisms, including bacteria, algae, and brine shrimp, and it can bind to substrates in the system (including activated carbon) (Cardeilhac and Whitaker 1988).
Still other factors can cause the copper concentration to rise too high. Increases in salinity will decrease the binding (adsorption) of copper to surfaces. In salt water at more neutral pH (e.g., pH of around 7), copper is surrounded by chloride molecules. Decreases in pH will release previously bound copper, and increase levels in solution, thereby increasing the risk of toxicity. Also, if some live foods, such as brine shrimp, are present during copper treatments, they may bioaccumulate enough copper to be toxic to fish that eat them (Cardeilhac and Whitaker 1988). (Additional factors are discussed in “Environmental Factors” below.)
CHELATED COPPER
Chelating agents are compounds added to copper sulfate in water. These agents help keep copper in solution by forming a ring-structured complex with copper. These complexes vary in their stability, depending upon the agent used. EDTA, one such agent, is very stable in solution. Citrate is also used, but citrate-copper complexes are less stable. However, citrate-copper complexes have more biological activity than EDTA-copper complexes, and are also easier to remove after treatment (Cardeilhac and Whitaker 1988).
In general, larger aquaculture facilities and public aquaria prefer to use copper sulfate rather than chelated copper complexes, because strength and activity of chelated copper complexes are more uncertain, and chelated copper compounds are also more difficult to remove.
COPPER TOXICITY TO TARGET ORGANISMS
At recommended Cu2+ concentrations of 0.15–0.20 mg/L, free copper is toxic to a number of organisms that are pathogens of fish, including the marine parasites Cryptocaryon irritans and Amyloodinium ocellatum. However, copper is effective primarily against the free-swimming, infective stages of these parasites—the Cryptocaryon theronts and the Amyloodinium dinospores (Cardeilhac and Whitaker 1988). Therefore, an understanding of the life cycle of these parasites is critical, and prolonged treatments (a minimum of 3–4 weeks for Cryptocaryon and 10–14 days for Amyloodinium) are generally required (Yanong 2009; Reed and Francis-Floyd 1994).
COPPER TOXICITY TO NON-TARGETED ORGANISMS
ANIMAL CONSIDERATIONS
Some species of fish are highly sensitive to copper and will die even at concentrations below therapeutic levels (i.e., less than 0.15 mg/L free copper). Other considerations that will affect survival include acclimation period (exposing fish to slowly increasing concentrations of free copper over the course of several days until the treatment target concentration is reached), as well as age or life stage of the fish. In one study, larvae acclimated to copper exposure more quickly than juvenile and adult fish and had better survival (Sellin et al. 2005). In some fish species, younger fish are more resistant to copper toxicity than older fish; in others, the reverse is true (Howarth and Sprague 1978; Pickering and Lazorchak 1995; Furata et al. 2008). Copper will damage a number of organs and systems, including the gills, liver, kidney, immune system, and nervous system (Cardeilhac and Whitaker 1988). Gills appear to be the most affected organ during acute toxicity, and will become blunt and thickened and lose ability to regulate body fluid ion concentrations. Copper also suppresses immune system function, and can affect the lateral line of fish. Prolonged copper exposure also may result in reduced growth (Wong et al. 1999). During toxicity, in addition to general signs of distress (e.g., increased respiration), fish may display darkening and behavioral abnormalities: lethargy, incoordination, problems with posture and balance, and, eventually, death (Cardeilhac and Whitaker 1988).
Most invertebrates are highly sensitive to copper and will not survive a copper treatment. If systems with invertebrates are to be treated, the invertebrates should be moved and not returned until Cu2+ concentrations are 0.01 mg/L or less, but ideally zero (Cardeilhac and Whitaker 1988). Copper levels should be monitored for some time after treatment, because copper bound to substrate (e.g., coral, shells, decorations) may be released if pH drops or other changes in water quality parameters occur (see Environmental Factors below).
ENVIRONMENTAL FACTORS
A number of factors will determine the toxicity of copper in water: a) the amount of free copper (Cu2+) in the water; b) the sensitivity of the fish or invertebrate species exposed; c) the age of the fish; d) the acclimation time to target concentration; e) the presence of substrates, especially those made of calcium or magnesium carbonate (including dolomite, oysters shell, and coral), that may remove copper from the water by adsorption; f) the presence of dissolved substances that may bind with copper and reduce its activity, including carbonates; g) the presence of “live foods” that may absorb and bioaccumulate (biologically concentrate) copper in their bodies; and h) the tank water pH (Cardeilhac and Whitaker 1988). Because copper levels can vary over time–for instance, they may suddenly increase with a drop in pH–copper concentration should be measured at least twice a day and adjusted accordingly (see section below).
BACTERIAL CONSIDERATIONS
Copper is also toxic to the nitrifying bacteria in the biofilter. At 0.3 mg/L Cu2+, copper sulfate inhibits ammonia and nitrite oxidation; therefore, increases in ammonia or nitrite levels in the system should be monitored closely during copper treatments. By contrast, bacteria that can cause disease in fish are much more resistant to copper, with some only inhibited or killed at free copper levels as high as 1.25 mg/L (Cardeilhac and Whitaker 1988).
DETERMINING COPPER DOSE (CONCENTRATION)
Copper sulfate (“bluestone” copper; blue copperas; copper sulfate pentahydrate) is 25.5% free copper (Cu2+), the active ingredient used to treat marine systems. Correct copper sulfate dosing is based on the free copper portion of the preparation; in marine systems, the recommended dose of Cu2+ for treatment of parasites, including Cryptocaryon sp. and Amyloodinium sp., is 0.15–0.20 mg/L Cu2+
To determine the grams (g) of copper sulfate estimated to be necessary to treat a given volume of water at a given desired concentration of free copper, use one of the formulas below:
If volume is known in gallons: Volume in gallons × 0.0038 (conversion factor) × (concentration of free copper desired in mg/L) × 3.92 = quantity required in grams
EXAMPLE 1 (gallons).
100-gallon tank; desired concentration of free copper: 0.15 mg/L
Formula (gallons): Volume in gallons × 0.0038 (conversion factor) × (concentration of free copper desired in mg/L) × 3.92 = quantity required in grams
100 × 0.0038 × 0.15 mg/L × 3.92 = 0.223 grams of copper sulfate pentahydrate needed
If volume is known in liters: Volume in liters × (concentration of free copper desired in mg/L) × 0.00392 = quantity required in grams
EXAMPLE 2 (liters).
1000-liter system; desired concentration of free copper: 0.20 mg/L
Formula (liters): Volume in liters × (concentration of free copper desired in mg/L) × 0.00392 = quantity required in grams
1000 × 0.20 mg/L × 0.00392 = 0.784 grams of copper sulfate pentahydrate needed
If using over-the-counter copper products, follow the manufacturer’s directions.
REACHING AND MAINTAINING DESIRED CONCENTRATIONS
When treating a tank of marine fish with copper, any materials or filtration components (e.g., activated carbon) that may bind copper should be removed; if necessary, organic loading and detritus should be removed. Baseline water quality parameters that should be measured prior to treatment include ammonia, nitrite, pH, temperature, alkalinity, and salinity. The recommended dose range of 0.15–0.20 mg/L free copper (Cu2+) should be reached gradually, over 2–3 days. This approach allows fish time to increase internal substances and physiological mechanisms that protect their bodies against toxicity, including the production of copper-binding proteins, such as metallothioneins (De Boeck et al. 2003).
Because water quality, substrates, and other factors determine measured levels of free copper, achieving any specific dose in a system can be challenging. After calculating the amount of copper needed (and always have your calculations checked by one or two others!), add half of the amount to the system. This can best be done by first mixing the copper sulfate with distilled water (as long as the volume of distilled water doesn’t drastically change the salinity of the system) and distributing half of the solution proportionately throughout each tank and the sump, avoiding the formation of “hot spots.”
Alternatively, the copper solution can be poured gradually into the sump; however, this acute, high-dose exposure may damage the biofilter by killing beneficial bacteria. After water in the system has cycled long enough that the copper should be evenly distributed, measure the free copper levels. Add more copper, allow time to mix, and re-measure. Do this until the desired concentration is reached.
Often, due to binding (adsorption) of copper to components of the system, more copper than the amount calculated initially will be needed to reach the appropriate concentration. Copper measurements should be taken twice a day, with more copper added if necessary. As discussed previously, treatment may last 3–4 weeks or more, depending upon the target organism and specific situation. Consult with a fish health specialist to determine duration of treatment and effectiveness.
REMOVING COPPER FROM THE SYSTEM
High quality, activated carbon effectively removes dissolved free copper from systems. One recommendation is to place a separate filtration unit containing fresh, activated charcoal at the rate of 170 grams per 57 liters of water (about 0.375 lbs per 15 gallons) on a system to be purged of copper (Cardeilhac and Whitaker 1988). Once all the water is believed to have cycled through the carbon, test for free copper concentration. If chelated copper has been used, water changes will be necessary. Dolomite may also be used, if it is removed afterward (Cardeilhac and Whitaker 1988). If tests continue to show a high free copper concentration, a complete water change may still be required to remove copper from the water. Copper levels should be monitored throughout this process and for several weeks afterward, in case copper that was previously bound to substrate or complexed in solution, is released as free copper.
SUMMARY
Before treating any system with copper, check local, state and federal regulations to ensure legal use.
Copper at a dosage rate of 0.15–0.20 mg/L Cu2+ is effective for control of important fish parasites, including Amyloodinium and Cryptocaryon, many species of algae, unwanted invertebrates, and fish parasites.
Copper sulfate (copper sulfate pentahydrate) is the most commonly used form of copper in marine aquarium and marine aquaculture systems. Because saltwater has greater ion content than freshwater, copper chemistry in marine systems is more complicated than in freshwater systems. In addition, many other factors affect the final concentration of free copper in water.
Copper can be toxic to some sensitive fish species and is highly toxic to many invertebrate species. Even for more tolerant species, chronic copper use can damage gills, kidneys, spleens, and other organs and systems. Copper will depress the immune system. Copper can also damage the beneficial bacteria in the biofilter.
Consult a fish health specialist during any disease outbreak or other situation for which you may consider using copper. If unsure about the effect on a given species, test on one or a few individuals before treating an entire group of fish. Invertebrates should be removed prior to treatment with copper.
Dosage calculation for use of copper in marine systems is different from that developed for use in freshwater systems, and is based on measured concentration of free copper ion (Cu2+). By contrast, in freshwater systems, measured alkalinity is normally used to calculate dosage rate (Watson and Yanong 2006). Copper sulfate pentahydrate (bluestone copper) is composed of 25.5% of the active ingredient (Cu2+) used to treat marine systems.
When dosing a system, therapeutic levels (0.15–0.20 mg/L) should be reached gradually over 2–3 days to allow fish to acclimate. Copper levels should be measured at least twice a day.
Activated carbon and water changes can be used to remove copper, once treatment is completed, but ideally, levels should be checked regularly for several weeks afterward, in case of copper leaching.
REFERENCES AND SUGGESTED READING
Cardeilhac, P.T. and B.R. Whitaker. 1988. Copper treatments: uses and precautions. In Tropical fish Medicine. Stoskopf, M.K., ed. The Veterinary Clinics of North America: Small Animal Practice 18(2): 435–448.
De Boeck, G., T.T.H. Ngo, K. Van Campenhout, and R. Blust. 2003. Differential metallothionein induction patterns in three freshwater fish during sublethal copper exposure. Aquatic Toxicology 65: 413–424.
Francis-Floyd R. and M.R. Floyd. 2011. Amyloodinium ocellatum, and important parasite of cultured marine fish. USDA Southern Regional Aquaculture Center Publication No. 4705. 12 pp.
Furata, T., N. Iwata, and K. Kikuchi. 2008. Effects of fish size and water temperature on the acute toxicity of copper for Japanese flounder, Paralichthys olivaceus, and red sea bream, Pagrus major. Journal of the World Aquaculture Society 39(6):766–773.
Howarth, R.S. and J.B. Sprague. 1978. Copper lethality to rainbow trout in waters of various hardness and pH. Water Research 12:455–462.
Pickering, Q.H. and J.M. Lazorchak. 1995. Evaluation of the robustness of the fathead minnow, Pimephales promelas, larval survival and growth test. Environmental Toxicology and Chemistry 14:653–659.
Sellin, M.K., E. Tate-Boldt, and A.S. Kolok. 2005. Acclimation to Cu in fathead minnows: Does age influence the response? Aquatic Toxicology 74(2): 97–109.
Watson, C.A. and R.P.E. Yanong. 2006. Use of Copper in Freshwater Aquaculture and Farm Ponds. FA008. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/FA008 (accessed November 18, 2009)
Wong, P.P. K., L.M. Chu, and C.K. Wong. 1999. Study of toxicity and bioaccumulation of copper in the silver sea bream Sparus sarba. Environment International 25(4): 417–422.
Yanong, R.P.E. 2009. Cryptocaryon irritans Infections (Marine White Spot Disease) in Fish. FA164. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/FA164


